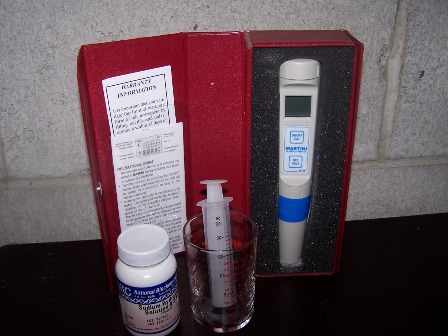
Testing for acid doesn’t have to cost a lot especially if you already have a pH meter . For the cost of a bottle of Sodium Hydroxide Solution (NaOH) either 0.1 or 0.2 normal $6, a plastic syringe marked in milliliters from the local drug store $2, and a small pyrex container from Walmart $1 you can make a very reliable and accurate acid titration test kit.
To do the test you simply measure out a known amount of wine (sample) into the container. For this test I will use 15 ml, this is about the smallest sample that will cover the probe on my meter. Draw out 10 ml of NaOH solution with the syringe. Insert the pH probe into the wine sample and slowly start adding NaOH solution swirling to mix. The pH will increase as you add the NaOH solution. When the pH reaches 8.2 stop and write down the amount of NaOH used. Now you have most of the information needed to plug into the formula and figure out the acid level of your wine.
The full formula for figuring out acid is; Acid = ((molecular weight of acid / number of H+ ions donated by each molecule of acid)*(volume of NaOH added)*(Normality of NaOH solution)) / (volume of wine sample).
Here are the Molecular Weight and H+ Ions of acids found on most wines and used in acid blend.
Acid Molecular Weight H+ Ions
Tartaric 150.09 2
Malic 134.09 2
Citric 210.14 3
Example: Lets say we used 6 ml of .2 NaOH solution to get a pH of 8.2 during the test. If we use the chart above we can figure out the acid in our wine. While Tartaric acid is the dominant acid in grape wines it is not always the Dominant Acid in Fruit wines. For a list of dominant acids in fruit wines I go to http://winemaking.jackkeller.net/acid.asp Jack Keller’s web site. Lets say we are making a Plum/Apple Blend wine. By looking at the chart on Jack’s site we see the dominant acid in Plum and Apple is Malic. So now we have all the numbers we need to plug into the formula.
Acid = ((molecular weight of acid / number of H+ ions donated by each molecule of acid)*(volume of NaOH added)*(Normality of NaOH solution)) / (volume of wine sample).
Acid = ((134.09 / 2)*6*0.2) / 15
Acid = (67.045*6*0.2) / 15
Acid = 80.454 / 15
Acid = 5.3636 g/L malic.
Now if this was a grape wine where tartaric acid is dominant then the formula would go like this:
Acid = ((150.09 / 2)*6*0.2) / 15
Acid = (75.045*6*0.2) / 15
Acid = 90.054 / 15
Acid = 6.0036 g/L tartaric.
There are a lot of shortcuts out there for this formula, and they work as long as you are using the right NaOH normally and the right size wine sample for that particular shortcut. If you use this formula and plug all the numbers in from scratch you will always have the correct results. In my opinion the only reason there is 0.2 normal NaOH is because as in the last example when used with a 15 ml sample on grape wine with Tartaric Acid dominant the number of ml of NaOH used also equals the Acid level.
I hope this isn’t to confusing, I think it’s good to know information when working with fruit wines which aren’t always tartaric acid dominant.

Pingback: TA Testing